
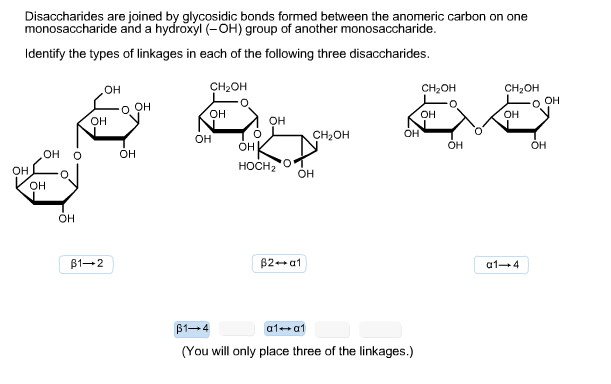
Oligosaccharide – a carbohydrate with a moderate (~ 3 to ~10) number of monosaccharide units bound together by glycosidic bonds. Mutarotation – the change in optical rotation that occurs when a pure anomer equilibrates to the different anomer through ring-chain tautomerism. Sucrose, by contrast, is a disaccharide which can be hydrolyzed into glucose and fructose. Glucose, ribose, mannose, and many other sugars (including deoxyribose) are monosaccharides. Monosaccharide – a sugar which cannot be hydrolyzed into simpler sugar units. L- (and D- ) When drawn in a Fischer projection,the highest-numbered chiral center in all L- sugars will be pointing to the left. Ketose – a monosaccharide with a ketone (or masked ketone) functional group. A hexose that bears an aldehyde (or masked aldehyde) is called an aldohexose a hexose bearing a ketone is called a ketohexose. Hexose – a sugar with six carbons, the most familiar example being glucose. Haworth Projection – a graphical depiction of monosaccharides that clearly shows the stereochemistry at each carbon (at the expense of accurately depicting its conformation). Glycosidic bond – the bond that links the anomeric carbon of a sugar to another compound. Formally, a glycoside is an acetal derived from condensation of the hemiacetal (or hemiketal) carbon of a sugar with the hydroxy group of another compound. Glycoside – fancy name for an acetal (or ketal) formed from a sugar. Furanoses are distinct from pyranoses, which is a six-membered cyclic hemiacetal. The name derives from furan, a five membered cyclic ether. įuranose – a five-membered cyclic hemiacetal. D-Glucose and D-Mannose are epimers, as are D-Mannose and D-Talose. The sugars of a disaccharide are joined through a glycosidic bond.Įpimer – two sugars which differ in the configuration of a single chiral center. Some prominent examples of disaccharides are lactose, sucrose, and maltose.
ĭisaccharide – a carbohydrate which can be hydrolyzed to give two monosaccharides. The simplest sugar is glyceraldehyde, which has one chiral center and exists in two enantiomeric forms, called D- and L- glyceraldehyde, respectively. The enantiomer of a D-sugar is always an L-sugar. If the hydroxyl group of this carbon is on the right, the sugar is assigned the prefix D- if it is on the left, it is assigned with the prefix L.
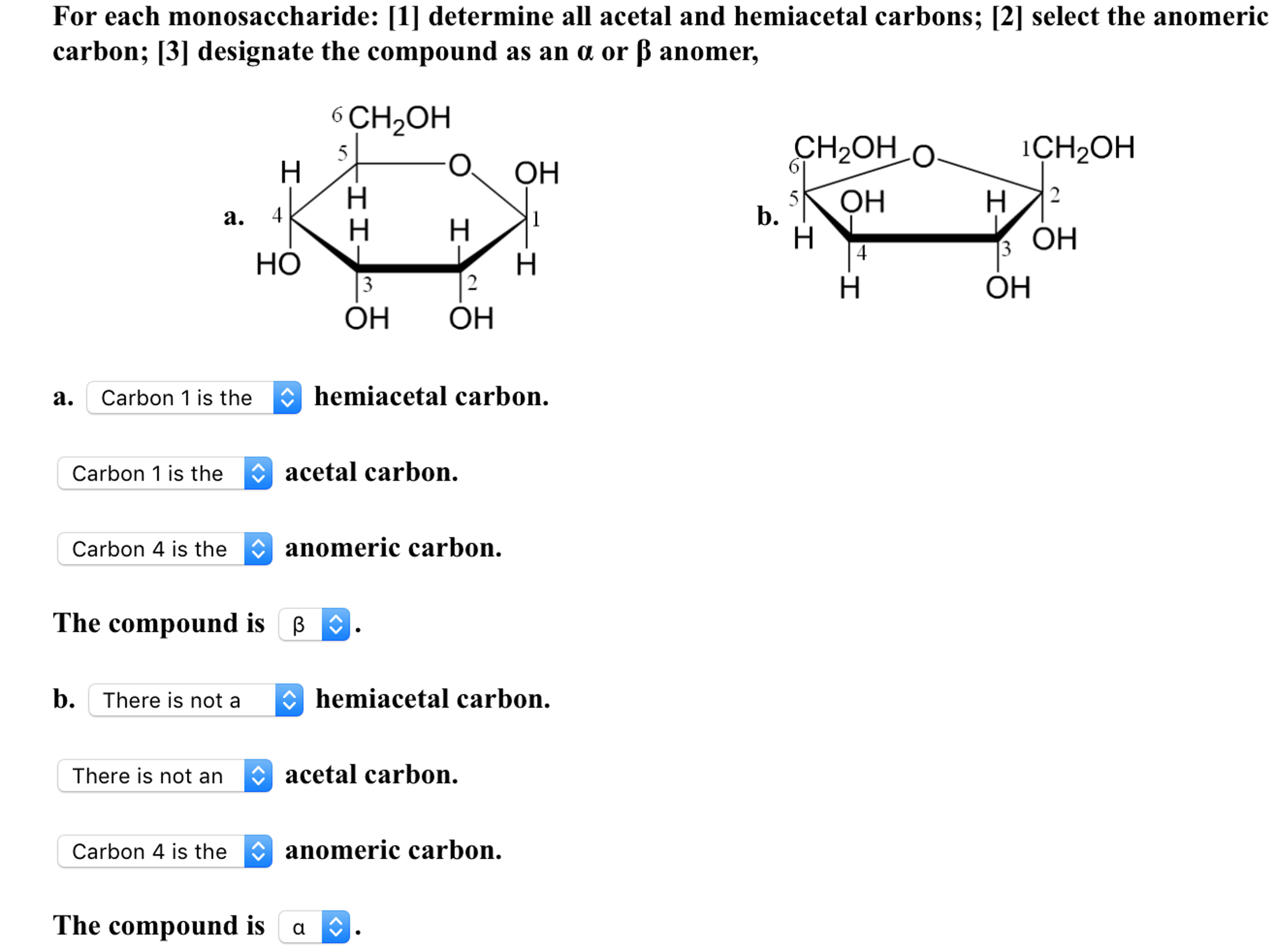
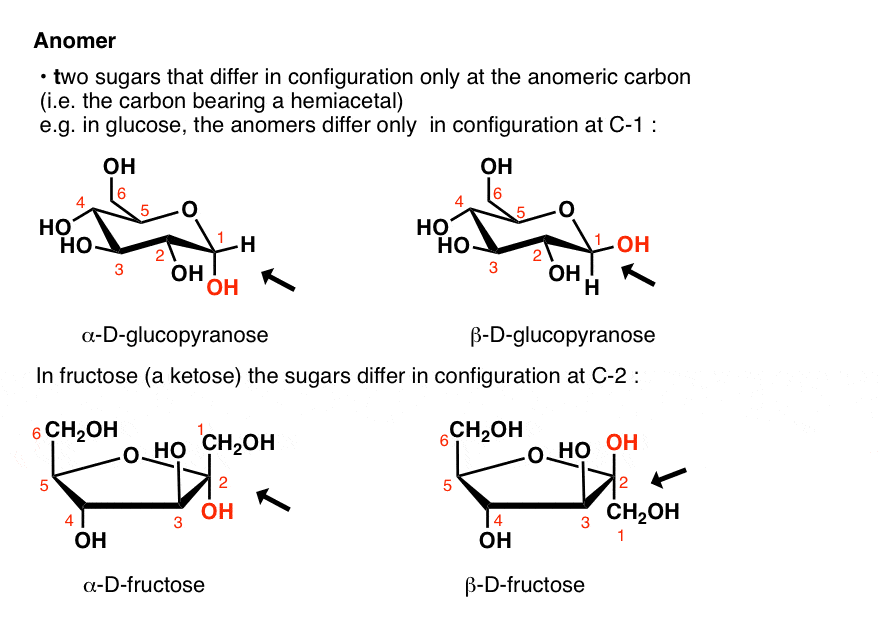
the bottom-most chiral center in its Fischer projection. Now used as a more generic term referring to mono-, oligo-, and polysaccharides as well as many of their derivatives.ĭ- (and L-) A monosaccharide is assigned to D- or L- according to the configuration at the highest-numbered chiral center, i.e. Contrast with alpha (α) where the two substituents are on the opposite faces of the ring.Ĭarbohydrate – originally just referred to monosaccharides (such as glucose) with the empirical formula C n(H 2O) n (i.e. Β (beta) – the name given to the configuration of a cyclic sugar where the oxygen on the anomeric carbon is on the same face of the ring as the substituent on the other carbon flanking the ring oxygen. This is C-1 in aldoses, and C-2 in the case of fructose. The two anomers are described with the terms α (“alpha”) and β (“beta”), defined above.Īnomeric carbon – the carbon of a cyclic sugar bearing a hemiacetal or acetal (hemiketal or ketal). If these names sound familiar, it is because alditols are commonly used as food additives and sweeteners.Īnomer – the name given to two diastereomeric monosaccharides that are epimers at the anomeric carbon. Sorbitol is the alditol of glucose, mannitol is the alditol of mannose, and so forth. The alditol of glyceraldehyde, for example, is glycerol. Contrast aldoses with ketoses, which are monosaccharides with a ketone (or masked ketone) group.Īlditol – an acyclic alcohol derived from the reduction of an aldose. from the action of nitric acid:Īldose – a monosaccharide with an aldehyde (or masked aldehyde) functional group. Contrasted with beta (β) which is where the two substituents are on the same faces of the ring.Īldaric acid – a dicarboxylic acid derived from an aldose where both ends of the sugar have been oxidized, e.g. Α (Alpha) – the name given to the configuration of a cyclic sugar where the oxygen on the anomeric carbon is on the opposite face of the ring relative to the substituent on the other carbon flanking the ring oxygen.

Feedback on anything that is missing would be greatly appreciated. Man, is there ever a lot of sugar nomenclature to know! This post aims to provide single place giving you the definitions of terms like anomeric carbon, alpha versus beta, pyranose versus furanose, and all the other most commonly encountered carbohydrate-related terms in a typical second-semester organic chemistry course.


 0 kommentar(er)
0 kommentar(er)
